Quality Assurance
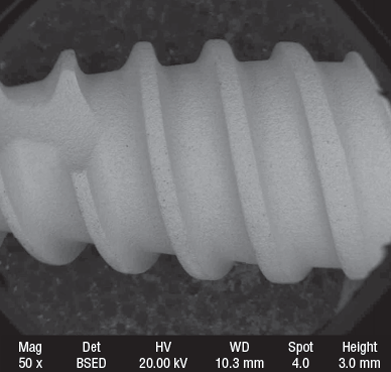
The GP Implants Quality System commitment is to ensure that GP Implants medical devices are with compliance to international standards and requirements.With the years of experience in the market, our company thrives to perfection in both quality assurance and customer services.R&D and Production ProcessTitanium Ti-s6Al-4V (Grade 5)Quality starts with selection of raw materials. All GP implant products are made from Titanium alloy 6Al4V and 6Al4V ELI. These alloys are currently the best titanium materials used in medical and dental orthopedics. These alloys are especially obtained by adding 6% Aluminum and 4% Vanadium metals to impart superior properties including high strength-to-weight ratio, very high corrosion resistance, high modulus of elasticity and excellent osseointegration. Human bone does not accept metallic objects easily. Titanium alloys 6AL4V and 6AL4V ELI used by GP implants have this special ability to allow bone to grow into the metal and form a strong natural bond, through a process known as osseointegration. ProfessionalismGP Implant Ltd has a state of the art computer numerically controlled (CNC) machinery specialized for implant manufacture. However, our high mark is our team of professionals who control and operate this set up. Our production operations are directed by a well-coordinated team of experienced physicians and professional engineers focusing on developing, designing and building innovative products for the medical field, especially dental implants and other dental appliances. We employ the most advanced manufacturing technologies that ensure maximum precision during manufacture, thus providing unmatched quality and reliability to all our products. Outstanding XPS & SEM resultsOur implants are treated with a large grit acid etching and sand-blasting process. Each and every implant is individually blasted with abrasive particles in an automated system which controls the speed, direction, pressure and size of the particles that collide with the implant. Repeatability is ensured, that is, each and every implant undergoes the same process and demonstrates the same excellent surface characteristics. Sand-blasting and acid etching (SLA) are done on implants to increase the surface area and roughness. This allows a larger contact area for better absorption of plasma proteins and blood into the implant micro-pores, thus ensuring faster osseointegration, and higher stability-essential qualities demanded of a good implant. Test resultsTo ensure quality of production our samples are subjected to an X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). These tests provide Nano level detail about the surface and structure of our products and confirm the excellent quality of our implants. Multipartite trials of GP ImplantsWe did not stop at XPS and SEM tests. Our implants were subjected to operational trials. A multi-centric study, which included universities and private practices, investigated GP Implants with SLA (sand-blasted and acid-etched) Surface. The implants were placed in the maxilla and the mandible. The study protocol included immediate loading 190 implants that were placed in 67 patients, with 40 implants placed in fresh extraction alveoli. After 6 months, only 8 implants in 5 patients had been lost, 6 in the mandible and 2 the maxilla. After 6 months under load 98.4% of the implants were optimally osseointegrated and fully functional. Your insuranceWe strive to stay abreast with all technological developments in the field. We’re also continually testing the components and geometries of the world‘s most prominent dental manufacturers, and always ensuring that we stay at the leading edge. PackagingA clean room, sometimes called dust-free room is a special enclosed volume where the number of particles of dust etc. is kept below a specified standard. An ISO class 7 clean room environment will permit no more than 107 particles of size over 0.1 micrometer in one cubic meter volume. For a comparison, typical city environment in a developed country contains 3.5 times as many particles which are at least 5 times larger, and many more, smaller particles. To prevent any contamination, our implants are packaged into vials (tubes) in our ISO Class 7 Clean Room without touching any material other than titanium. We use packaging media from suppliers maintaining extremely high quality standards. The range of materials available includes transparent bags, blister packaging, roll bags, and PS tubes with plugs (for dental implants). We also provide the following services: • Specialized packaging; SterilizationOne of the key elements of our supply chain system is our close cooperation with prominent radiation sterilization suppliers. Gamma radiation sterilization is currently the best sterilization technique for sealed packages. It ensures that there are no organisms are present in the sealed vials containing the implants.
SLA SURFACE TREATMENT• SURVIVAL RATES High and consistent survival rates (over 97.6% after a five-year follow-up). |